You’re due to go in for a medical procedure. But after what happened at a UCLA hospital, you’re a little apprehensive.
Two patients died at the Ronald Reagan UCLA Medical Center in a superbug CRE outbreak, caused by two medical scopes that still carried the bacteria even after they were disinfected.
In addition to the two victims, seven hospital patients were infected with the deadly superbug between October and January. The medical center has contacted 179 others who had endoscopic procedures between October and January and is offering them home tests to screen for the bacteria.
The superbug, carbapenem-resistant Enterobacteriaceae, or CRE, can kill up to half the patients who contract them, the Centers for Disease Control and Prevention says.
So, should you cancel your procedure?
Here’s what you need to know:
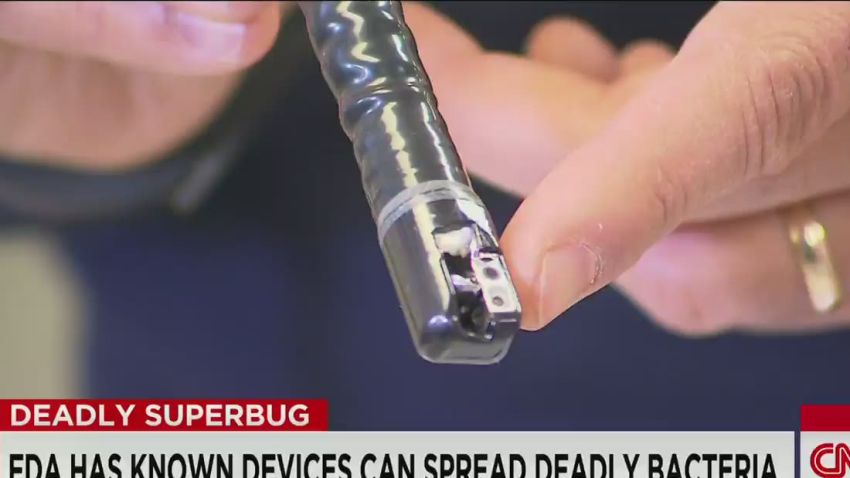
1. What type of equipment caused these horrible infections?
They’re called duodenoscopes.
The UCLA hospital was using a duodenoscope made by Olympus Corp. of the Americas, but the Food and Drug Administration is also reviewing data from the two other U.S. companies that make the devices, Fujifilm USA and Pentax Medical.
Duodenoscopes are most commonly used for procedures on the gallbladder, pancreatic ducts, and the bile ducts, which are a series of thin tubes that reach from the liver to the small intestine.
2. I’m scheduled to get a colonoscopy soon. Should I be worried?
No. Duodenoscopes are not used for colonoscopies.
3. How common are these infections, and why do they happen?
More than half a million duodenoscope procedures are done every year in the United States, and there have been fewer than 100 known cases of transmission of the CRE bacteria, according to the American Society for Gastrointestinal Endoscopy.
The problem is this: A part of the scope called “the elevator” can be tough to clean because it has many small moving parts.
According to the FDA, the cleaning instructions that come with duodenoscopes say to brush the elevator area – but that might not be enough.
“The moving parts of the elevator mechanism contain microscopic crevices that may not be reached with a brush,” the FDA said Thursday. “Residual body fluids and organic debris may remain in these crevices after cleaning and disinfection. If these fluids contain microbial contamination, subsequent patients may be exposed to serious infections.”
4. Yech. I’m supposed to have a procedure with a duodenoscope. Should I cancel it?
No. A procedure with a duodenoscope can be lifesaving. It can remove gallstones, for example, or insert a stent into a blocked bile duct. If you need it, you need it.
5. OK. My doctor says I need it. So how do I make sure I’m safe?
Remind your doctor that following the manufacturer’s cleaning instructions on a duodenoscope might not be enough.
Show your doctor this advisory from the FDA that recommends additional cleaning practices, including meticulously cleaning the elevator mechanism by hand. Many hospitals already do this.
Also, show your doctor this article from the Centers for Disease Control: A hospital in Illinois put a stop to duodenoscope infections by using a technique called gas sterilization. Other hospitals have started testing their scopes for bacteria and only using them when the results come back negative.
CNN’s Debra Goldschmidt also contributed.