Story highlights
The cell-phone-size device is for monitoring and treating type 1 diabetes
The device is for patients 14 and older and should be available by the spring
The Food and Drug Administration approved a so-called artificial pancreas Wednesday. The first-of-its-kind device, the size of a cell phone, monitors and treats patients with type 1 diabetes, also known as juvenile diabetes.
In those with type 1 diabetes, the pancreas does not produce enough insulin, a hormone people need to get energy from food. The Medtronic MiniMed 670G system continuously monitors glucose (blood sugar) levels and delivers needed insulin to patients.
“This is a revolutionary day for the treatment of diabetes. We’ve been long awaiting the artificial pancreas, and it’s exciting to see it,” said Dr. Robert Courgi, an endocrinologist at Northwell Health’s South Side Hospital in Bay Shore, New York.
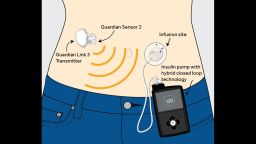
The device, which requires a prescription and will become available during the spring, is intended for patients 14 or older, according to the company’s website.
The Medtronic system includes a glucose meter (an electrode under the skin), an insulin pump strapped to the body and an infusion patch connected to the pump, with a tiny catheter for delivering insulin. The system measures a patient’s glucose levels every five minutes and either administers or withholds insulin as needed, helping patients maintain glucose levels within the normal range the majority of the time.
Type 1 diabetes
Only 5% of people with diabetes have the type 1 form of the disease, for which there is no cure, according to the Juvenile Diabetes Research Foundation.
Patients with the autoimmune disorder must regularly monitor their blood-sugar levels, inject or continually infuse insulin through a pump, and carefully balance insulin doses with both their eating and physical activities. Failure to do so can result in either too high or too low concentrations of blood sugar, either of which could lead to a coma.
As explained by Courgi, patients with type 1 diabetes currently have access to glucose-monitoring systems, which can measure blood sugar levels. However, they have to perform the next step of taking a dose of insulin.
The newly approved device will not only measure their glucose but also administer the insulin for them, Courgi said, adding, “it certainly makes things much easier.”
“It is designed to learn what an individual’s insulin needs are and to take action to minimize both high and low glucose levels,” said Hooman Hakami, group president of Medtronic Diabetes, in a blog post.
Although little or no input is required of a patient to maintain basal insulin – also referred to as “background” insulin, this is the amount available whether a person eats or not – the artificial pancreas does require users to manually adjust their bolus insulin.
Bolus insulin refers to the extra doses of insulin a normally functioning pancreas makes in response to eating food. Type 1 diabetes patients currently take a bolus dose of insulin to respond to the natural spike in blood sugar that can occur after eating certain foods.
FDA concerns
As part of the approval process for the Medtronic device, the FDA evaluated data from a clinical trial involving 123 participants.
“The fear was life-threatening hypoglycemia (low glucose levels) from the device,” said Courgi, who heard a lecture on the artificial pancreas at an endocrinology conference last year. He has no ties to the company that makes it.
However, no serious side effects, including severe hypoglycemia, were reported during the study. However, the FDA still requires further study to better understand how the device performs in real-world settings once it is commercially available.
Join the conversation
Medtronic, based in Dublin, Ireland, has begun studies to evaluate the safety and effectiveness of the device when used by diabetic children between the ages of 7 and 13.
The FDA cannot be faulted for beginning with older patients first, said Courgi. “We expect improvements … as it becomes more mainstream and more data comes back; then we can extend it to the younger population.”

