Story highlights
A new study found an Ebola vaccine protected 100% of people who received it
The strategy used to deliver it was inspired by the strategy to eliminate smallpox
An experimental vaccine against the Ebola virus was found to be 100% effective, according to a study published in The Lancet on Thursday.
The results offer hope of better protection against the disease that ravaged West Africa in 2014, killing more than 11,000 people.
“Ebola left a devastating legacy in our country. We are proud that we have been able to contribute to developing a vaccine that will prevent other nations from enduring what we endured,” said Dr. KeÏta Sakoba, the director of the national agency for health security in Guinea.
The vaccine
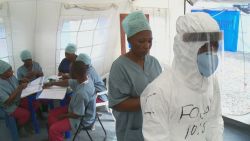
The experimental vaccine was given in 2015 to people in Guinea who were in contact with patients who had recently confirmed cases of Ebola.
A few months after the early trials, the World Health Organization said the preliminary results were an “extremely promising development.”
Initially, only people over the age of 18 were offered the vaccine and the participants receiving them were randomized.
But the process was stopped after initial results in order to get the vaccine to everyone in need of it.
The trial involved more than 11,000 people, according to the WHO who led the trial in conjunction with Guinea’s Ministry of Health.
When analyzing the results, the teams didn’t count people who got sick within the 10 days, as they were believed to have been infected before they received the vaccine. Waiting 10 days also gave volunteers time to build up an immunity after receiving the vaccine, according to Dr. Marie-Paule Kieny, the WHO assistant director-general and the study’s lead author.
Patients were either vaccinated immediately or after three weeks. As everyone had become eligible for the vaccine, the three-week group served as the control as they didn’t actually get the vaccine until it was clear that they were not infected (Ebola has an incubation time of two to 21 days), Kieny said.
Researchers followed up with immunized volunteers at their homes on days three, 14, 21, 42, 63 and 84 after receiving the vaccine.
A total of 5,837 people were given the rVSV-ZEBOV vaccine, and none had a recorded case of Ebola after 10 days or longer, the study says.
Among people who were not immediately given the vaccine, there were 23 cases.
Some people who had the vaccine reported headaches, fatigue and muscle pain. Two patients had serious reactions, including one who had an allergic reaction.
There are multiple strains of the Ebola virus, and this vaccine covers the Zaire group and offers cross-protection for similar strains in this group, Kieny said. But it doesn’t not confer protection from all strains of the virus, nor from the related, and lethal, Marburg virus.
Other vaccines are also being studied, Kieny said.
We won’t be defenseless
Ebola was first discovered in 1976, and before the 2014 outbreak, it typically hit isolated African communities – those outbreaks were much more manageable for medical teams to parachute in and treat patients.
But the virus reached cities in 2014, spreading like wildfire and catching the global health community off guard.
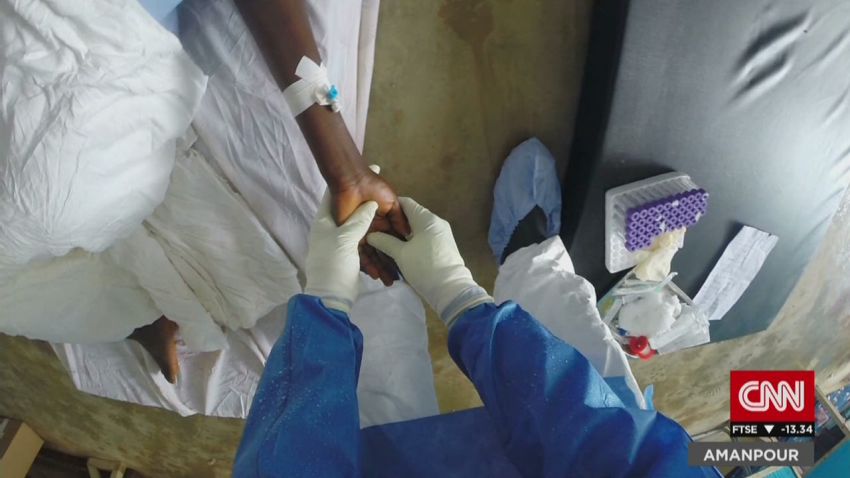
Ebola is highly contagious once patients are symptomatic, so as families and close-knit communities tried to care for sick loved ones, they risked infection themselves. A vaccine, however, can protect them.
“The principle is to stop transmission,” Kieny said. “So you are one step quicker than the transmission of the disease.”
To enable this, researchers doled out the vaccine to so-called “clusters” or “rings” – groups of people who had been in contact with an Ebola patient.
It’s the same strategy that was used to eradicate smallpox.
“The premise is that by vaccinating all people who have come into contact with an infected person, you create a protective ‘ring’ and stop the virus from spreading further,” John-Arne Rottingen of the Norwegian Institute of Public Health, which has been involved in implementing the trial, told CNN last year.
The vaccine was developed in Canada but is now owned and manufactured by Merck, Sharp & Dohme. It’s currently being fast-tracked by US and European regulatory agencies.
A new vaccine takes about 10 years, on average, to become available, according to Kieny.
Merck has promised to ensure that 300,000 doses of the vaccine will be available in case of a new Ebola flareup. It will submit the vaccine for licensing by the close of 2017.
“When the next Ebola outbreak hits, we will not be defenseless,” Kieny said.